Effects of diclofenac, aceclofenac and meloxicam on the metabolism of proteoglycans and hyaluronan in osteoarthritic human cartilage
https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1038/sj.bjp.0703710
Abstract
- Since nonsteroidal anti‐inflammatory drugs (NSAIDs) may impair the ability of the chondrocyte to repair its damaged extracellular matrix, we explored the changes in the metabolism of newly synthesized proteoglycan and hyaluronan (HA) molecules produced by aceclofenac, diclofenac and meloxicam in human osteoarthritic (OA) cartilage.
- Explants were sampled from the medial femoral condyle and were classified by use of the Mankin's histological‐histochemical grading system. Cartilage specimens exhibited moderate (M) OA in 20 subjects and had severe (S) OA in 20.
- Cartilage explants were pulsed with [‐3H]‐glucosamine and chased in the absence or in the presence of 0.3–3 μg ml−1of either aceclofenac, diclofenac or meloxicam. After papain digestion, the labelled chondroitin sulphate ([‐3H]‐proteoglycans) and [‐3H]‐HA molecules present in the tissue and media were purified by anion‐exchange chromatography.
- In cartilage with MOA and SOA, the metabolic balance of proteoglycan and HA was unaffected by diclofenac. In contrast, and in a dose‐dependent manner, aceclofenac and meloxicam both increased the synthesis of proteoglycans and HA in explants with MOA and SOA; these two NSAIDs also reduced significantly the net loss of [‐3H]‐proteoglycans and [‐3H]‐HA molecules from cartilage explants.
- The data obtained in short‐term in vitrocultures indicate that, at the concentrations found in synovial fluid, aceclofenac and meloxicam may exert a favourable effect on the overall metabolism of proteoglycans and HA in cartilage with MOA and SOA.
British Journal of Pharmacology (2000) 131, 1413–1421; doi:10.1038/sj.bjp.0703710
Extract:
Effects of NSAIDs on the total synthesis of proteoglycan and HA
In both groups, diclofenac at the three concentrations tested (Figure 3, upper left panel) as well as aceclofenac at a concentration of 0.3 μg ml−1 (Figure 3, middle left panel) and meloxicam at the concentrations of 0.3 and 1 μg ml−1 (Figure 3, lower left panel) did not change significantly the total amounts (tissue and medium) of newly synthesized proteoglycan molecules. In contrast, a significant increase in proteoglycan synthesis was observed when cartilage explants from two groups were incubated with either aceclofenac at the concentrations of 1 and 3 μg ml−1 or with meloxicam at a concentration of 3 μg ml−1 (P=0.002). In each group, the increase in proteoglycan synthesis was always stronger when explants were incubated with aceclofenac than with meloxicam (P=0.002). Further comparison between explants with MOA and SOA disclosed that the increase in proteoglycan synthesis was significantly higher in explants with less advanced OA lesions at a meloxicam concentration of 3 μg ml−1 (P<0.001) and at an aceclofenac concentration of 1 μg ml−1 (P<0.001) and 3 μg ml−1 (P<0.001).
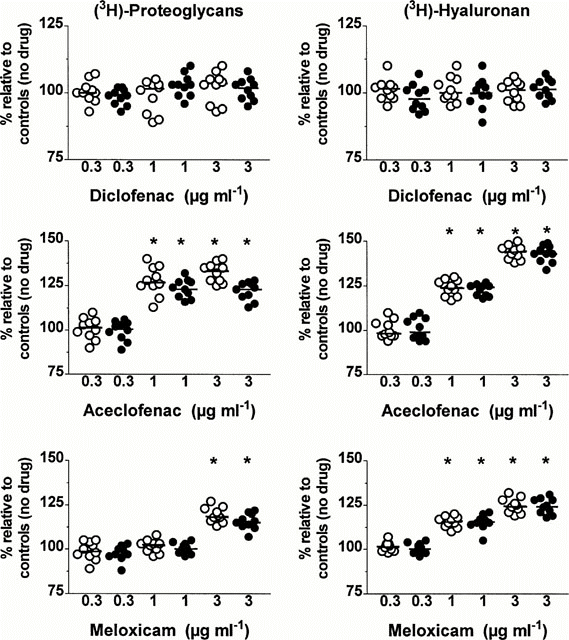
Figure 3
Effects of different concentrations (μg ml−1 of diclofenac, aceclofenac and meloxicam on the total amounts (tissue+medium) of newly synthesized proteoglycan and hyaluronan molecules in cartilage explants with moderate (open circles) and severe (closed circles) osteoarthritis. Results are expressed as the negative percentage (%) of values observed in corresponding explants cultured in the absence of drug. The horizontal line present in each column scatter corresponds to the median value. *P: comparison by the Wilcoxon signed‐rank test.
In explants with MOA and SOA, the total amounts of newly synthesized HA molecules were unaffected by diclofenac at a concentration of 0.3, 1 and 3 μg ml−1 (Figure 3, upper right panel) as well as by 0.3 μg ml−1 of either aceclofenac (Figure 3, middle right panel) or meloxicam (Figure 3, lower right panel). On the other hand, at the concentrations of 1 and 3 μg ml−1, both aceclofenac and meloxicam enhanced HA synthesis in a relatively dose‐dependent manner in the two groups (P=0.002). The increase in HA synthesis observed at these two concentrations was however stronger with aceclofenac than with meloxicam (P=0.002) and this in the two groups. No statistically significant difference in the increase of HA synthesis could be disclosed between the two groups at the concentrations of 1 and 3 μg ml−1 of either aceclofenac or meloxicam.
Discussion
The data presented herein are the first to describe the effects of aceclofenac, diclofenac and meloxicam on the metabolism of HA in explants of human OA cartilage.
Cartilage composition and metabolism vary widely in the different topographical areas of the same joint and between the different joints of the same individual (Muir, 1986; Holmes et al., 1988). Therefore, the articular tissue was sampled from the same region of the knee joint to restrict variations in the concentration and metabolism of both proteoglycan and HA. Further, as cartilage composition and metabolism also change with age, the donors had a very similar range of age distribution in both groups, so that differences observed in response to NSAIDs could be more closely related to the OA disease process rather than ageing.
The concentrations of the three NSAIDs used in our culture system are similar to the range of total (bound and free) concentrations of these drugs that has been observed in human synovial fluid (Bort et al., 1996; Turck et al., 1996). It is however difficult to assess the actual effective concentration of drug acting on the tissue since this depends upon various factors such as the pH of the synovial fluid, the integrity of the surface of the cartilage, the partition coefficient of the drug in the articular tissue as well as protein binding. Most NSAIDs are extensively bound to plasma proteins and our culture media contained a relatively small proportion (20%) of foetal calf serum. Therefore, it is likely that, at the total concentration of 3 μg ml−1, the concentration of the free drug in our culture media was higher than that present in synovial fluid bathing cartilage in vivo.
Diclofenac unaffected the HA metabolism of OA cartilage, whereas, in a dose dependent manner, both aceclofenac and meloxicam were able concomitantly to increase HA synthesis and reduce the loss of newly synthesized HA molecules from the articular tissue. Further, the action of aceclofenac was stronger than that of meloxicam. That these two NSAIDs had a positive effect on the metabolic balance of HA is worth stressing since the progressive reduction in the HA content of OA cartilage (Manicourt et al., 1988; Rizkalla et al., 1992) is likely to contribute, at least in part, to the apparent irreversibility of the OA disease process (Pita et al., 1992) and further contrasts with the age‐related increase in the HA content of normal articular cartilage (Holmes et al., 1988).
Our knowledge of the synthesis and degradation of HA in articular cartilage is still fragmentary. Chondrocytes do express two different HA synthases (HAS) which are localized to the cell surface, but the enzymatic characteristics of the two HAS isoforms as well as their mechanisms of regulation, including the possible effects of NSAIDs, remain to be clarified (Hiscock et al., 2000). On the other hand, as no hyaluronidase has been identified thus far in the cartilage matrix, it has been suggested that the degradation and loss of HA molecules may result from the action of oxygen‐derived free radicals (ODFR) (Ng et al., 1995). Although NSAIDs may block the production of ODFR (Minta & Williams, 1985), it is likely that no single factor accounts for the favourable effect of aceclofenac and meloxicam on the overall metabolism of HA in the OA cartilage. Therefore, the exact mechanisms of action of the two drugs should be elucidated in further studies as they might be of great biological and therapeutic significance in OA.
Aceclofenac and meloxicam also reduced the loss labelled proteoglycan molecules from the articular tissue and both drugs concomitantly enhanced proteoglycan synthesis whereas diclofenac unaffected the overall metabolism of sulphated glycosaminoglycans. Previous in vitro studies have indeed shown that, at concentrations within therapeutic range, the effect of NSAIDs on the ability of chondrocytes to synthesize proteoglycans may be either stimulatory, inhibitory or neutral (Brandt, 1987; Dingle, 1999). Therefore, it is likely that a mechanism other than COX inhibition accounts for these marked differences in the effects of these drugs on proteoglycan synthesis, a contention further strengthened by the report that the PG E1 analogue, misoprostol, does not protect against the suppression of proteoglycan synthesis caused by NSAIDs (Brandt et al., 1991). Inhibition of IL‐1 production and consequent expression of growth factor activity have been recently proposed as possible stimulatory mechanisms (Dingle, 1999). On the other hand, some NSAIDs do have toxic effects on chondrocyte metabolism such as inhibition of glucuronyltransferase, an enzyme responsible for the elongation of chondroitin sulphate chains on the nascent proteoglycan molecules (Hugenberg et al., 1993).
That meloxicam enhanced the rate of proteoglycan synthesis contrasts with the report of Rainsford et al. (1997) who found that this drug unaffected cartilage proteoglycan production. The reasons for this apparent discrepancy are unknown, but it is possible they reflect, in part, differences in the histological‐histochemical grade of cartilage explants. Indeed, Dingle (1999) has reported that, in contrast to OA cartilage, normal cartilage shows no evidence of proteoglycan stimulation with aceclofenac. This heightened susceptibility of pathological articular cartilage has already been observed in vivo and in vitro with several NSAIDs (Brandt, 1987). Although its exact mechanism is unknown at present, data in the literature suggest that the uptake of NSAID by cartilage is inversely related to the proteoglycan content of the matrix (Brandt, 1987). Accordingly, any decrease in the concentration of the negatively charged proteoglycans, which is proportional to the gravity of the OA process, would increase the permeability of the matrix to the acidically charged NSAID.
The reduction in the net loss of proteoglycans produced by aceclofenac and meloxicam might be, at least in part, related to the positive effect of these drugs on the overall metabolism of HA since any decrease in the HA content of cartilage is likely to limit the aggregation of proteoglycans and, in so doing, favours the loss of newly synthesized proteoglycan molecules by diffusion or by proeolytic degradation (Heinegard & Hascall, 1974). On the other hand, reports have shown that, at concentrations within the therapeutic range, several NSAIDs inhibit the proteoglycanase and collagenase activities present in OA cartilage (Vignon et al., 1992; Barracchini et al., 1998). It remains to determine whether this suppressive effect of NSAIDs on the loss of proteoglycans is due either to an inhibition of the production of ODFR (Halliwell, 1995), or to a reduction in the synthesis of metalloproteinases (MMPs) and other proteolytic enzymes and/or to a stimulation of the synthesis and secretion of tissue inhibitors of proteolytic enzymes (Poole et al., 1995). Recent studies also suggest that NSAIDs could act either as reversible enzymatic inhibitors (Barracchini et al., 1998) or by inhibiting COXs which mediate the induction of membrane‐type metalloproteinase‐1 (MMP‐14), an enzyme able to activate gelatinase A (MMP‐2) and collagenase‐3 (MMP‐13) (Takahashi et al., 1999).
In conclusion, although it remains to be established whether changes observed in cartilage metabolism over short‐term in vitro cultures would also occur in vivo as a result of long‐term administration, the results presented herein show that, in contrast to diclofenac, aceclofenac and, to a lesser extent, meloxicam at the concentrations found in synovial fluid both exert a favourable effect on the overall metabolism of proteoglycans and HA in OA cartilage. Accordingly, the two drugs should not hamper the biomechanical properties of the articular tissue and might delay joint failure in OA. Aceclofenac and meloxicam, however, did not normalize the changes in cartilage metabolism seen in the OA tissue.